|
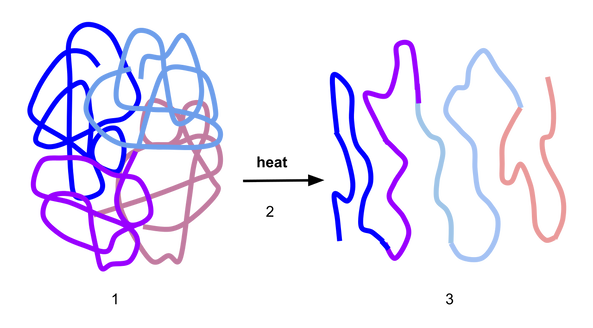
Children are still enjoying the summer holidays, but the schools are due to re-open their doors in September and I’m very conscious that I will soon be inundated with calls from concerned parents and letters from GPs or practice nurses with the same old question I get every year. “My child has an allergy to egg; can they have the flu vaccine?” and “this child needs a flu vaccine to be administered in a hospital due to having an egg allergy.” The fact is that there are very few individuals who cannot receive any influenza vaccine. The Department of Health guidelines state that none of the influenza vaccines should be given to those who have had: either a confirmed anaphylactic reaction to a previous dose of the vaccine, difficult to manage asthma, or a confirmed anaphylactic reaction to any component of the vaccine. So, if your child has none of these, then they can have their annual flu vaccine. If you are still concerned, talk to your GP. If an egg-free vaccine is not available, they should be able to advise you about a suitable vaccine with very low egg content. Sometimes, based on a child’s previous medical history, your GP may refer your child to the hospital to have a flu vaccine. This requires prior consultation with an allergy consultant and when appropriate, booking a day in the hospital where the child will be given a skin prick test. If the test is negative, part of the vaccine is given first and after a period of observation, the rest of the vaccine is administered. A little science: With egg allergy (as with all food allergies), we are concerned about the body’s mistaken immune response to the proteins in the egg which are: ovalbumin, ovotransferrin, ovomucoid, ovomucin and lysozyme. Ovalbumin is the major protein in egg white and it’s heat resistant - meaning that even when processed - sometimes egg still cannot be tolerated by individuals with egg allergy. Other egg proteins such as ovomucoid can be denatured by the heat. As a result, the shape of the protein changes (see the figure below) and increases the likelihood of tolerance. Inactivated influenza vaccines are egg-free or have extremely low ovalbumin content. For example, the Quadrivalent Influenza Vaccine (split virion, inactivated) manufactured by Sanofi Pasteur®, contains 0.12 micrograms/ml which is equivalent of a 0.05 microgram in 0.5 ml dose. Evidence shows that even LAIV manufactured by Fluenz Tetra® which previously had a higher content of ovalbumin of 1.2 micrograms/ml was also safe when given to children with egg allergy. LAIV now contains a significantly reduced ovalbumin content of a £ 0.024 microgram in a 0.2 ml dose. The Green Book is a document issued by the Department of Health about vaccines and every year, an update is issued about available vaccines and all relevant guidelines applicable to those vaccines. Your GP should have received their copy and will able to offer the best vaccine for your child. References:
Comments are closed.
|
AuthorAneta Ivanova Archives
March 2023
Categories |
The Consulting Rooms, 38 Harborne Road, Birmingham, B15 3EB
[email protected]
Website design & content by LIT Communication: www.litcommunication.com
[email protected]
Website design & content by LIT Communication: www.litcommunication.com

 RSS Feed
RSS Feed